What is flow cytometry?
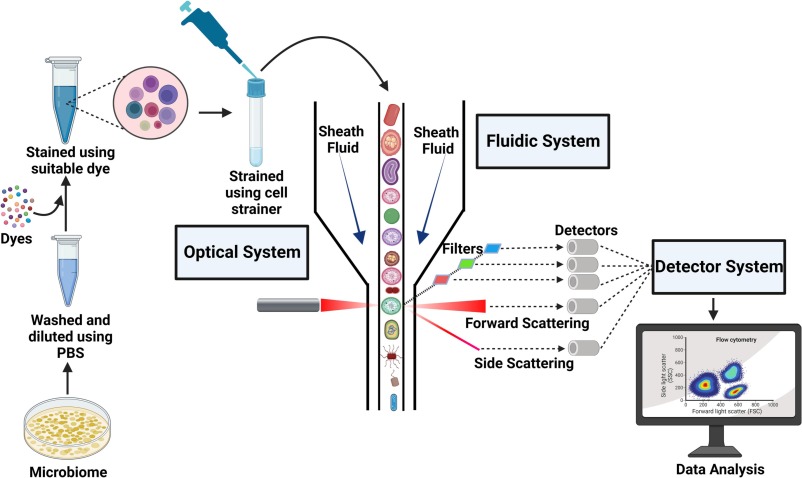
Flow cytometry is a laser-based technique used to analyze and quantify cells or particles as they flow in a fluid stream through a beam of light. The technology measures multiple parameters simultaneously, including cell size, granularity, and fluorescence intensity, which can be used to identify and characterize specific cell populations.
The core components of a flow cytometer include:
- Fluidics System: Suspends and transports cells in a stream to the laser intercept.
- Optics System: Consists of lasers and detectors to illuminate cells and capture scattered and emitted light.
- Electronics System: Converts light signals into digital data for analysis.
- Software: Analyzes and visualizes the data, often in the form of scatterplots or histograms.
During the process, cells are typically labeled with fluorescent dyes or antibodies conjugated to fluorochromes, which bind to specific cell surface markers or intracellular molecules. As cells pass through the laser, they scatter light and emit fluorescence, which is detected and quantified.
Key applications of flow cytometry
Flow cytometry has a wide range of applications across research and clinical settings:
1. Immunophenotyping
Flow cytometry is widely used to identify and characterize immune cells based on their surface markers. This is crucial for understanding the immune system’s role in health and disease, such as in autoimmune disorders, infections, and cancer.
2. Cancer Diagnosis and Monitoring
In hematologic malignancies like leukemia and lymphoma, flow cytometry helps classify cancer cells based on their unique marker expression. It is also used to monitor minimal residual disease (MRD) after treatment.
3. Cell Sorting
Fluorescence-activated cell sorting (FACS), a specialized application of flow cytometry, allows researchers to physically separate cell populations based on their fluorescence and scatter properties. This is invaluable for isolating specific cell types for further study.
4. Cell Cycle and Apoptosis Analysis
Flow cytometry can assess DNA content to determine cell cycle stages and detect apoptotic cells, providing insights into cellular mechanisms and drug effects.
5. Functional Assays
The technology can measure intracellular cytokine production, calcium flux, and reactive oxygen species, enabling researchers to study cell function and signaling pathways.
6. Stem Cell Research
Flow cytometry is used to identify and isolate stem cells based on their surface markers, aiding in regenerative medicine and developmental biology studies.
7. Microbiology
In microbiology, flow cytometry can analyze bacteria, yeast, and other microorganisms, providing information about their viability, size, and metabolic activity.
Advantages of flow cytometry
- High Throughput: Thousands of cells can be analyzed per second, making it a rapid and efficient technique.
- Multiparametric Analysis: Multiple parameters can be measured simultaneously, providing comprehensive data.
- Sensitivity: Fluorescent labeling allows for the detection of rare cell populations or low-abundance molecules.
- Versatility: Applicable to a wide range of sample types, including blood, tissues, and cultured cells.
Limitations of flow cytometry
Despite its many advantages, flow cytometry has some limitations:
- Cost: Flow cytometers and reagents can be expensive, limiting access for some laboratories.
- Complexity: Data analysis requires expertise, and the interpretation of multiparametric data can be challenging.
- Sample Preparation: Proper labeling and handling of samples are critical to obtaining accurate results.
Flow cytometry applications in Gastroenterology and Hepatology
Flow cytometry has found significant applications in the field of Gastroenterology and Hepatology, where it is used to study gastrointestinal (GI) diseases, immune responses, and cellular mechanisms. Its ability to analyze complex cell populations and detect subtle changes in cell function makes it a valuable tool for both research and clinical diagnostics in GI disorders. Below is an elaboration on the use of flow cytometry in gastroenterology:
1. Inflammatory Bowel Disease (IBD)
Inflammatory bowel diseases, such as Crohn’s disease and ulcerative colitis, are characterized by chronic inflammation of the GI tract. Flow cytometry is widely used to study the immune cells involved in these conditions.
- Immune Cell Profiling: Flow cytometry can identify and quantify immune cell subsets (e.g., T cells, B cells, macrophages, and dendritic cells) in the intestinal mucosa and peripheral blood. This helps researchers understand the role of specific immune cells in driving inflammation.
- Cytokine Production: Intracellular cytokine staining allows researchers to measure the production of pro-inflammatory cytokines (e.g., TNF-α, IL-6, IL-17) by immune cells, providing insights into the inflammatory pathways involved in IBD.
- Monitoring Therapy: Flow cytometry can be used to monitor the effects of biologic therapies (e.g., anti-TNF agents) on immune cell populations and cytokine profiles, helping to assess treatment efficacy.
2. Celiac Disease
Celiac disease is an autoimmune disorder triggered by gluten ingestion, leading to damage in the small intestine. Flow cytometry is used to study the immune response in celiac disease.
- T-Cell Analysis: Flow cytometry can detect gluten-specific T cells in the blood or intestinal mucosa, which play a key role in the pathogenesis of celiac disease.
- Intraepithelial Lymphocytes (IELs): Increased numbers of IELs are a hallmark of celiac disease. Flow cytometry can characterize these cells and their activation status.
- Autoantibody Detection: While not directly a flow cytometry application, bead-based assays using flow cytometry can detect autoantibodies such as anti-tissue transglutaminase (tTG) and anti-endomysial antibodies (EMA), which are diagnostic markers for celiac disease.
3. Gastrointestinal Cancers
Flow cytometry is used in the study and diagnosis of GI cancers, including colorectal cancer, gastric cancer, and esophageal cancer.
- Tumor-Infiltrating Lymphocytes (TILs): Flow cytometry can analyze TILs to understand the immune response to tumors and predict patient outcomes.
- Circulating Tumor Cells (CTCs): Detection of CTCs in the blood of GI cancer patients can provide prognostic information and help monitor treatment response.
- Minimal Residual Disease (MRD): In hematologic malignancies associated with the GI tract (e.g., lymphomas), flow cytometry can detect MRD after treatment.
4. Functional GI Disorders
Flow cytometry is increasingly being used to study functional GI disorders, such as irritable bowel syndrome (IBS), where immune activation and low-grade inflammation may play a role.
- Mast Cell Activation: Flow cytometry can quantify mast cells and measure their activation status, which is thought to contribute to IBS symptoms.
- Immune Cell Phenotyping: Researchers use flow cytometry to study changes in immune cell populations in the gut mucosa of IBS patients.
5. Infections of the GI Tract
Flow cytometry is used to study the immune response to GI infections caused by bacteria, viruses, or parasites.
- Helicobacter pylori: Flow cytometry can analyze the immune response to H. pylori, the bacterium responsible for gastritis and peptic ulcers.
- Viral Infections: In viral gastroenteritis (e.g., rotavirus or norovirus), flow cytometry can measure changes in immune cell populations and cytokine production.
- Parasitic Infections: Flow cytometry can be used to study the immune response to parasites such as Giardia or Cryptosporidium.
6. Intestinal Barrier Function
The integrity of the intestinal barrier is critical for preventing the translocation of harmful substances into the bloodstream. Flow cytometry can be used to study cells involved in maintaining barrier function.
- Epithelial Cells: Flow cytometry can analyze intestinal epithelial cells and their expression of tight junction proteins, which are essential for barrier integrity.
- Immune Cell Interactions: Researchers can study how immune cells interact with epithelial cells to maintain or disrupt barrier function.
7. Microbiome and Immune System Interactions
The gut microbiome plays a crucial role in GI health and disease. Flow cytometry is used to study how the microbiome interacts with the immune system.
- Dendritic Cells and Macrophages: These cells are key mediators of microbiome-immune system interactions. Flow cytometry can characterize their phenotype and function.
- Regulatory T Cells (Tregs): Flow cytometry can quantify Tregs, which play a role in maintaining tolerance to the gut microbiota and preventing inflammation.
8. Organoid and 3D Culture Studies
Organoids, which are 3D cell cultures that mimic the structure and function of the GI tract, are increasingly used in gastroenterology research. Flow cytometry is used to analyze cells derived from organoids.
- Cell Differentiation: Flow cytometry can assess the differentiation of stem cells into various cell types within organoids.
- Drug Screening: Organoids can be treated with drugs, and flow cytometry can be used to evaluate their effects on cell viability, proliferation, and function.
9. Autoimmune Gastritis
Autoimmune gastritis is a condition where the immune system attacks the stomach lining, leading to inflammation and atrophy. Flow cytometry is used to study the immune cells involved in this process.
- T-Cell and B-Cell Analysis: Flow cytometry can identify autoreactive T and B cells that contribute to the destruction of gastric parietal cells.
- Cytokine Profiling: The production of inflammatory cytokines by immune cells can be measured to understand the underlying mechanisms.
10. Liver Diseases
While primarily focused on the GI tract, flow cytometry is also used to study liver diseases, which are often interconnected with GI disorders.
- Hepatitis: Flow cytometry can analyze immune cell populations in viral hepatitis (e.g., hepatitis B and C) or autoimmune hepatitis.
- Non-Alcoholic Fatty Liver Disease (NAFLD): Researchers use flow cytometry to study the immune response and inflammation in NAFLD.
Future directions
Advancements in flow cytometry continue to expand its capabilities. Spectral flow cytometry, for example, allows for the detection of an even greater number of fluorochromes simultaneously, enhancing multiparametric analysis. Mass cytometry (CyTOF) replaces fluorochromes with metal isotopes, enabling the analysis of over 40 parameters at once. Additionally, the integration of artificial intelligence (AI) and machine learning is improving data analysis and interpretation.
Conclusions
Flow cytometry is a cornerstone of modern biomedical research and clinical diagnostics. Its ability to provide detailed, multiparametric analysis of cells and particles has deepened our understanding of biology and transformed the way diseases are diagnosed and treated. As technology continues to evolve, flow cytometry will undoubtedly remain at the forefront of scientific discovery and medical innovation, paving the way for new breakthroughs in health and medicine.
In Gastroenterology and Hepatology, flow cytometry has become an essential tool, providing insights into the immune mechanisms underlying GI diseases, aiding in diagnosis, and guiding treatment strategies. Its ability to analyze complex cell populations and detect subtle changes in cell function makes it invaluable for both research and clinical applications. As the technology continues to advance, flow cytometry will likely play an even greater role in understanding and managing GI disorders, ultimately improving patient outcomes.
