Colorectal Cancer Screening: Comparing Cell-Free DNA and Multitarget Stool DNA Tests
Colorectal cancer (CRC) remains a significant public health challenge, ranking as the third most diagnosed cancer in the United States. While early detection can prevent the majority of CRC-related deaths, many eligible individuals remain unscreened. Innovations in screening technologies, such as cell-free DNA (cfDNA) blood tests and next-generation multitarget stool DNA tests, promise to enhance screening adherence, early cancer detection, and potentially reduce mortality rates.
Cell-Free DNA Blood-Based Test for Colorectal Cancer Screening
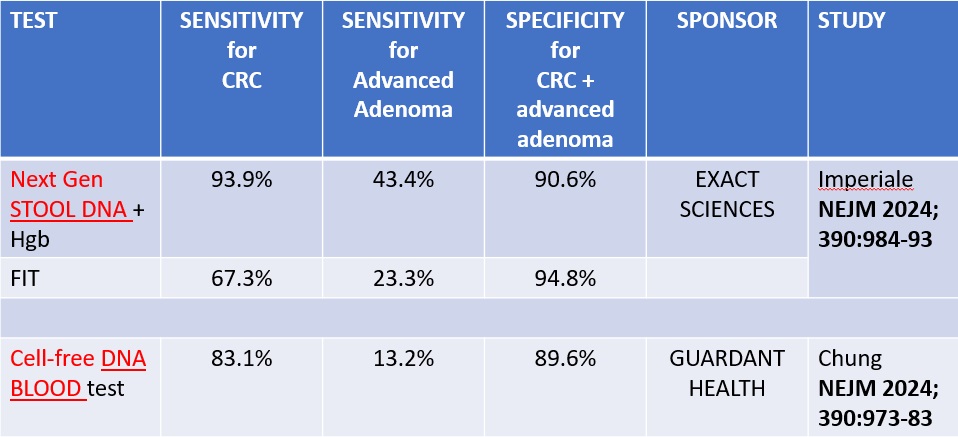
The cfDNA test aims to offer a minimally invasive option for early detection. According to recent studies, this test has demonstrated a sensitivity of 83.1% for detecting colorectal cancers, with specificity for advanced neoplasia (cancer or advanced precancerous lesions) at 89.6%. These figures are compelling, particularly when considering the test’s 87.5% sensitivity at detecting early-stage (I-III) cancers. However, it is less effective in identifying advanced precancerous lesions, with a sensitivity of only 13.2%.
Next-Generation Multitarget Stool DNA Test
Conversely, the next-generation stool DNA test incorporates assessments of DNA molecular markers and hemoglobin levels to improve performance metrics over existing stool-based tests. It shows a higher sensitivity for colorectal cancer at 93.9%, and for advanced precancerous lesions at 43.4%. Its specificity for advanced neoplasia is comparable at 90.6%, and it outperforms the cfDNA test in detecting early cancerous changes. When compared to the fecal immunochemical test (FIT), the next-generation test demonstrates superior sensitivity for both colorectal cancer and advanced precancerous lesions, albeit with a slight decrease in specificity for advanced neoplasia.
Comparative Analysis and Discussion
Both the cfDNA blood-based test and the next-generation stool DNA test provide robust options for CRC screening with different strengths. The cfDNA test, with its high specificity and good sensitivity for colorectal cancer, could be particularly useful for those who prefer a blood draw over stool-based tests. It might be more appealing due to its simplicity and non-invasiveness, potentially increasing compliance among screening-averse populations.
On the other hand, the stool DNA test’s superior sensitivity for both colorectal cancer and precancerous lesions makes it a highly effective screening tool, especially in identifying earlier stages of cancer that may be missed by other tests. However, its slightly lower specificity could lead to a higher number of false positives, which may result in unnecessary follow-up procedures.
Conclusion
Both screening tests mark significant advancements in the detection of colorectal cancer and offer promising alternatives to traditional methods like colonoscopy and FIT. The choice between these tests should consider factors such as patient compliance, risk level, and the specific screening goals of gastroenterologists. Continued improvements and comparative studies will be essential to fully integrate these technologies into mainstream screening protocols, aiming to reduce the burden of colorectal cancer through early detection and timely treatment.
References
- Imperiale TF, Porter K, Zella J, Gagrat ZD, Olson MC, Statz S, Garces J, Lavin PT, Aguilar H, Brinberg D, Berkelhammer C, Kisiel JB, Limburg PJ; BLUE-C Study Investigators. Next-Generation Multitarget Stool DNA Test for Colorectal Cancer Screening. N Engl J Med 2024 Mar 14;390(11):984-993.
- Chung DC, Gray DM 2nd, Singh H, Issaka RB, Raymond VM, Eagle C, Hu S, Chudova DI, Talasaz A, Greenson JK, Sinicrope FA, Gupta S, Grady WM. A Cell-free DNA Blood-Based Test for Colorectal Cancer Screening. N Engl J Med 2024 Mar 14;390(11):973-983.
