Gastric cancer: The role of gastric intestinal metaplasia (GIM)
Gastric cancer (GC) remains a significant global health challenge, ranking as the fifth most common cancer and the third leading cause of cancer-related deaths worldwide. Despite advancements in medical treatments, the lethality of GC is alarmingly high, with over 760,000 deaths attributed to it annually. This staggering statistic underscores the urgent need for deeper research into the mechanisms driving gastric cancer development and progression.
The Correa Pathway: From Inflammation to Cancer
The pathogenesis of GC, particularly the intestinal type, is believed to follow a sequential process known as the Correa pathway (See Figure below). This model proposes that GC originates from a background of chronic inflammation, primarily driven by a long-standing infection with the bacterium Helicobacter pylori (H. pylori). Chronic gastritis induced by H. pylori infection gradually leads to the atrophy of gastric epithelial cells, including acid-secreting parietal cells, setting the stage for the emergence of cells with intestinal characteristics.
Helicobacter pylori (H. pylori) plays a pivotal role in the pathogenesis of gastric cancer, particularly as a primary instigator of chronic gastritis, which often progresses to more severe gastric conditions such as atrophic gastritis, gastric intestinal metaplasia (GIM), and eventually gastric cancer. This bacterium colonizes the gastric mucosa and induces a persistent inflammatory response, which can disrupt the normal gastric environment. The inflammation caused by H. pylori infection is characterized by the infiltration of various immune cells into the gastric lining, leading to the production of cytokines and chemokines that perpetuate chronic inflammation. Over time, this ongoing inflammatory response contributes to the degradation of the gastric mucosal barrier, atrophy of gastric glands, and ultimately, the emergence of metaplastic and dysplastic changes in the gastric epithelium.
The relationship between H. pylori infection and gastric cancer is further underscored by the bacterium’s ability to induce genetic and epigenetic changes within the host cells. H. pylori has been shown to interact with gastric epithelial cells in a way that promotes mutations and alters gene expression, contributing to cellular transformation and carcinogenesis. Additionally, H. pylori can affect the expression of genes involved in cell cycle regulation, apoptosis, and DNA repair, further enhancing the risk of malignant transformation. Given these factors, the eradication of H. pylori has been a significant focus in the prevention of gastric cancer, with numerous studies demonstrating that eliminating the infection can significantly reduce the incidence of precancerous lesions and, consequently, lower the risk of developing gastric cancer. This underscores the critical importance of early detection and treatment of H. pylori infection as a strategic approach to prevent the progression of gastric pathology towards cancer.
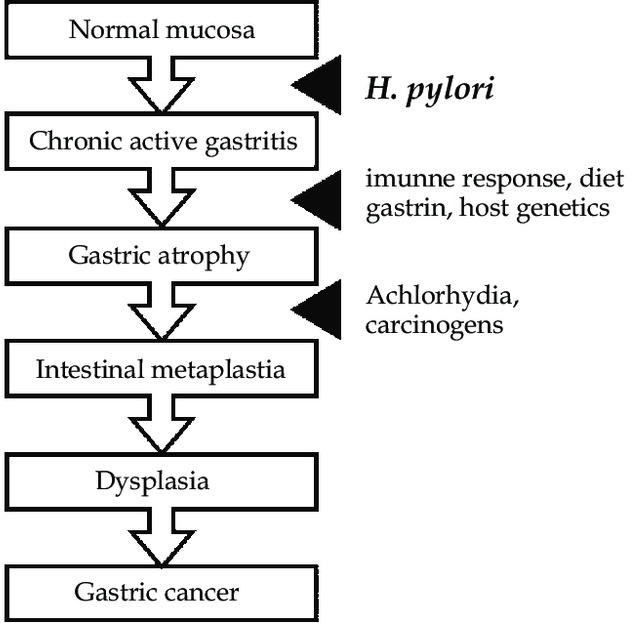
Gastric Intestinal Metaplasia (GIM): A Precursor to Gastric Cancer
Gastric Intestinal Metaplasia (GIM) is a critical condition in the Correa pathway where normal gastric epithelial cells transform into intestinal-type cells. This metaplastic change is a defense mechanism against chronic inflammation but comes with increased risks of malignancy. GIM is identifiable histologically by the presence of goblet cells and can be further characterized by the expression of intestine-specific markers like MUC2, CDX1, and CDX2.
Pyloric Metaplasia and Its Implications
In the progression towards gastric cancer, a significant transformation occurs in the gastric corpus, where the normal glandular structure is replaced by pyloric gland-like features, referred to as Spasmolytic Polypeptide-Expressing Metaplasia (SPEM). These changes are indicative of pyloric metaplasia, a condition closely linked to the loss of parietal and chief cells due to chronic inflammation.
Research and Future Directions
The study of GIM not only helps in understanding the early changes that precede gastric cancer but also serves as a potential target for early intervention. Research has shown that interventions targeting the molecular pathways involved in the transition from GIM to gastric cancer, such as those controlled by the transcription factors CDX1 and CDX2, may offer new preventive and therapeutic opportunities.
The relationship between GIM and GC highlights the importance of early detection and the potential for therapeutic interventions that could inhibit the progression from metaplasia to malignancy. Ongoing research is essential to unravel the complex molecular mechanisms underlying GIM and to develop strategies that could effectively intercept the progression towards gastric cancer.
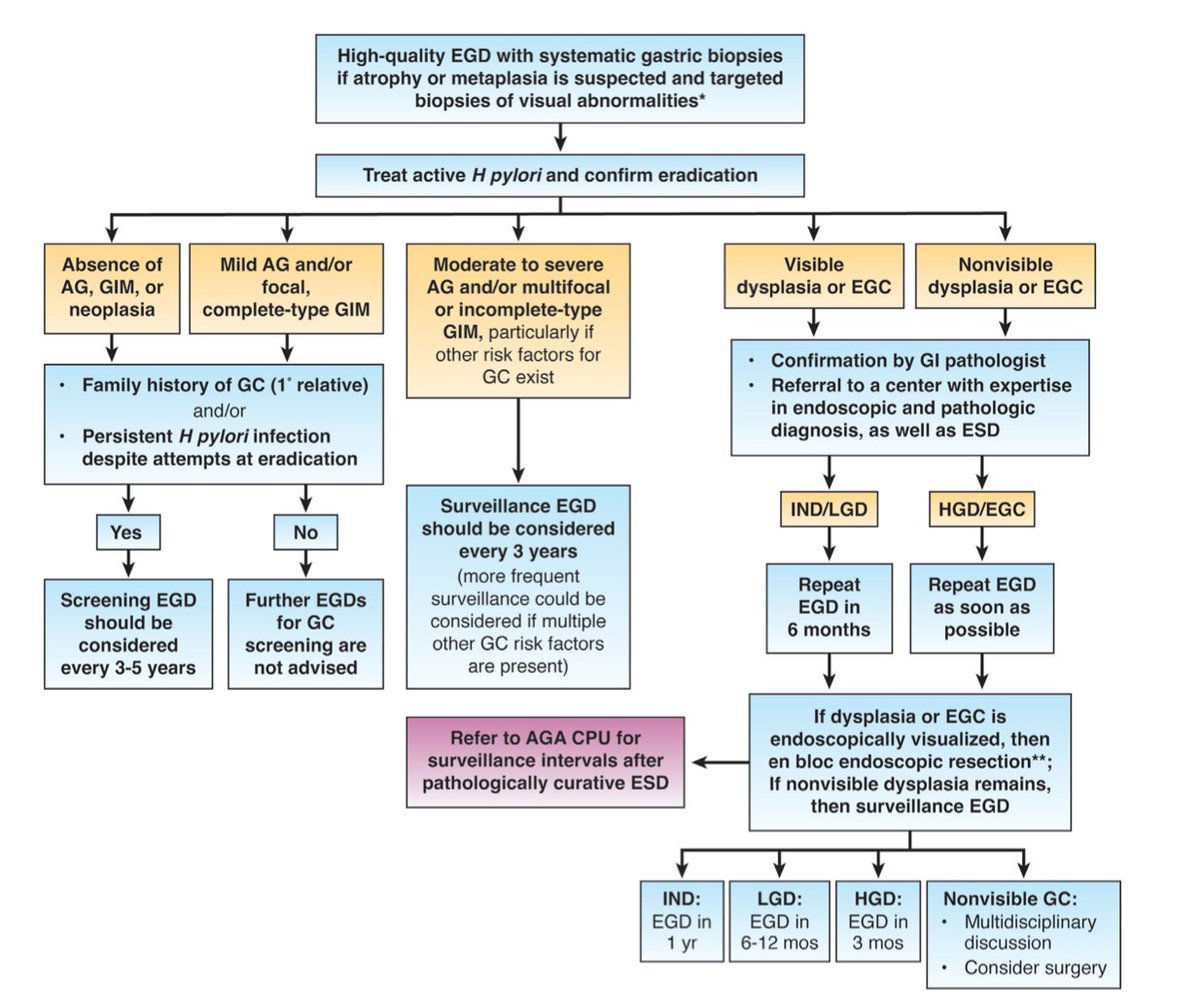
What to do when you find precancerous conditions such as atrophic gastritis or intestinal metaplasia?
In the following algorithm you can find the latest guideline on the follow up and management of the patients diagnosed histologically with atrophic gastritis, intestinal metaplasia or dysplasia.
Conclusion
As the global burden of gastric cancer continues to pose a significant challenge, understanding the underlying mechanisms of its development, such as those involving gastric intestinal metaplasia, is crucial. With continued research and advancements in molecular biology, there is hope for developing more effective prevention strategies and therapeutic interventions that could significantly reduce the incidence and mortality associated with this devastating disease.
Reference
- Shah SC, Wang AY, Wallace MB, Hwang JH. AGA Clinical Practice Update on Screening and Surveillance in Individuals at Increased Risk for Gastric Cancer in the United States: Expert Review. Gastroenterology 2025 (in press)
